laboratory endotoxin analysis|problems with endotoxin tests : mfg Streamline Your BET with Endosafe Cartridge TechnologyTraditional endotoxin testing has always had its challenges - long turnaround times, extensive training, potential for human error, and multi-step assay preparation. In addition, an . One of the world's leading online gambling companies. The .
{plog:ftitle_list}
GSEXY ACOMPANHANTES CAMPINAS. O Gsexy é o site adulto mais tradicional e conhecido da cidade, estamos na internet a mais de 16 anos e todas as acompanhantes Campinas que anunciam aqui passam por um processo rigoroso para divulgar as suas fotos, portanto não aceitemos anúncios com imagens fake. Por isso a nossa .
Researchers today have a handful of options for bacterial endotoxin testing (BET), each with their own pros and cons with respect to ease-of-use, sensitivity, cost, and regulatory considerations. The Limulus amebocyte lysate, or LAL endotoxin test, is by far the most .

Contaminant control. Drugmakers test injected products for bacterial endotoxins—lipopolysaccharides from the outer membranes of gram-negative bacteria. The .Streamline Your BET with Endosafe Cartridge TechnologyTraditional endotoxin testing has always had its challenges - long turnaround times, extensive training, potential for human error, and multi-step assay preparation. In addition, an .
In the US, the main guidance is found in the FDA's Guidance for Industry: Pyrogen and Endotoxins Testing: Questions and Answers.. Much of the information contained in the original 1987 FDA Guide is now covered by USP .
Multi-Cartridge Endotoxin Testing System. The Endosafe ® nexgen-MCS ™ supports the ability to test multiple endotoxin samples at once, via both Endosafe ® LAL and Trillium ™ recombinant cascade reagent (rCR) cartridges. The . Introduction. Endotoxin Testing Laboratories are crucial for ensuring the safety of pharmaceuticals and medical devices. This comprehensive guide delves into the principles, methods, and importance of endotoxin testing, highlighting the role of endotoxin testing labs in maintaining product safety and compliance with regulatory standards.The LAL test is now a standard method for endotoxin testing of parenteral drugs and medical devices and is specified as a harmonised method in the US, European and Japanese Pharmacopoeias (USP chapter 85, EP 2.6.14 Supplement 6.6 and JP General Test 4.01). . for multiple tests, or in bulk for laboratories handling large numbers of samples .
Microbiology Testing Laboratory offering identification of mold, bacteria, allergens, pathogens, mycotoxins, endotoxins, bed bugs, legionella, MRSA, Clostridium .STERIS provides contract analysis of bacterial endotoxins using methods compliant with EP, USP and ANSI/AAMI ST72 to meet FDA and MHRA requirements. Testing is performed according to USP , USP and ANSI/AAMI ST72. . STERIS Laboratories uses the chromogenic, kinetic turbidimetric and gel clot testing method. Bacterial endotoxin testing.What is Bacterial Endotoxin Testing? The Bacterial Endotoxins Test (BET) is an in vitro assay for detection and quantitation of bacterial endotoxins, a component of the cell wall of gram-negative bacteria. The BET is performed as part of the lot release testing for medical devices with direct or indirect contact to the cardiovascular system, lymphatic system, or cerebrospinal fluid.
Endotoxin testing is a critical quality control measure employed primarily in the pharmaceutical and medical device industries to ensure the safety and efficacy of products, especially those that come into direct contact with the human body. Endotoxins, primarily lipopolysaccharides (LPS) from the outer membrane of Gram-negative bacteria, can cause .Sensitive endotoxin testing is essential because of the sample limitations and low endotoxin levels required for cell culture and animal research. . Cooper JF, Levin J, Wagner HN Jr. (1971) Quantitative comparison of in vitro and in vivo methods for the detection of endotoxin. J Lab Clin Med 78:138–148. Seki N, Muta T, Oda T et al. (1994 .Expert endotoxin testing services for accurate results with our state-of-the-art microbiology lab. Experience the reliability of our USP bacterial endotoxins test. . Pace ® is accredited to ISO/IEC 17025, the international standard for competence in testing and calibration laboratories, validating our technical competence and commitment to . This is why endotoxin contamination of injectable products may provoke a severe and potentially even life-threatening reaction in the recipients. Endotoxin contamination testing in lab settings. Several different methods for endotoxin detection exist, among which the Limulus amebocyte lysate (LAL) assay is most widely established.
The Pierce Chromogenic Endotoxin Quant Kit uses the amebocyte lysate method to measure the interaction of endotoxins with proenzyme Factor C, which is sequestered from the amebocytes of horseshoe crabs. The proteolytic activity of this proenzyme is activated in the presence of LPS. Endotoxin levels are determined by measuring the activity of Factor C in the presence of the .Our global laboratories offer testing in water, soil, sludge, sewage and sediment to identify dangerous pathogens that may put human health at risk. We analyze a diverse range of materials and liquids to identify common bacteria and molds. Our services include characterization, enumeration, contamination source evaluation, and impact assessment .
why is endotoxin testing important
As bacterial endotoxins can pose health and safety hazards to patients, USP <85> requires bacterial endotoxin testing to detect and quantify the presence of endotoxins from Gram-negative bacteria in sterile compounds. To assure . The water may have had direct product impact or contact before any lab analysis is executed. Delays in testing only increase the amount of potential product impact – in the event of a failed test. . Another common problem is overwhelming the distillation purification process with a high level of endotoxin in the water going to the still . Additionally, we will discuss different methods of endotoxin testing and how they can be implemented in your laboratory or production environment. Why is Endotoxin Testing Important? Endotoxin testing plays .The Microbial Solutions Customer Portal is a dedicated location to store and access documents relating to your Endosafe and Celsis ® products, such as Endosafe CoAs for LAL reagents, Endosafe SDS, accessory products and cartridges, warranty forms, and package inserts.
Circle sample cutter agency
endotoxin testing Endotoxin Testing – Doing the right thing ? . Immunobiology Section . Laboratories Branch, TGA. 24 July 2019. Presentation Plan • Regulation of bacterial endotoxins • Brief history of pyrogens, endotoxins – testing and regulation • TGA involvement with validation, training, operator qualification Bioburden testing helps maintain laboratory standards as per the rules or protocols followed. . microorganisms. Whereas endotoxin testing detects and quantifies non-viable bacteria as dead gram-negative bacteria release endotoxin. Endotoxin is performed in sterile and nonpyrogenic devices or equipment, usually, that come in direct or indirect .
EnviroBiomics is a leading commercial environmental laboratory in USA that specializes in analyzing samples to determine the presence of mold, bacteria, and other microorganisms (including ERMI, HERTSMI-2, Actino testing, Mycotoxins, Endotoxins, etc.).. We collaborate with environmental and medical experts who make crucial decisions about the indoor .
Paper Dust Tester agency
when is endotoxin testing required
Gel-Clot (Pros): The accepted USP standard for Bacterial Endotoxins testing, Gel-Clot testing is the simplest, fastest, cheapest, and oldest of the 3 methods, and works by the production of a clot by Limulus Amoebocyte Lysate when in the presence of endotoxin. The Lysate reacts with endotoxin to cleave the protein coagulogen into coagulin .Microchem Laboratory specializes in the testing of disinfectants, sanitizers, antimicrobial devices, medical devices, personal care products, and dietary supplements. It also supports companies with a variety of process validations and microbial monitoring of critical environments. For routine testing in the quality control laboratory, it remains of vital importance to establish a reliable and reproducible method of testing so that any reaction can be examined to determine if the cause is indeed due to endotoxin or to .
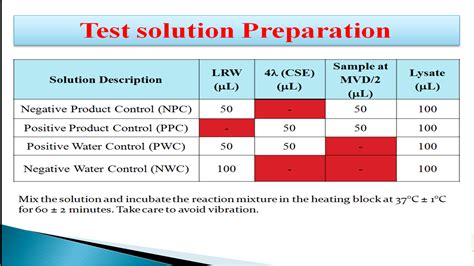
Case I: No rabbits show an individual rise of 0.6℃ in the temperature, i.e., the sum of the increase in temperature in the three rabbits does not exceed 1.4℃. It means the absence of pyrogen. Case II: If two or three rabbits show a rise in temperature of ≥0.6℃ or the sum of temperature rise exceeds 1.4℃. It indicated the presence of pyrogen in the sample.
The Next Generation of Endotoxin and Glucan Analysis Software for glass tube and plate readers from your Endotoxin experts. Associates of Cape Cod, Inc. introduces the next generation of endotoxin and glucan detection analysis software that offers integrated solutions for your quantitative endotoxin and glucan detection testing, reporting needs .Bacterial Endotoxin Testing Solutions . Charles River Laboratories Subject: Bacterial Endotoxin Testing Solutions Technical Sheet Keywords: BET, Bacterial Endotoxin test, LAL Test, Kinetic chromogenic, KCA, LAL reagents, Endosafe-PTS LAL cartridges, Endochrome-K, Kinetic turbidimetric, KTA KTA2, gel-clot, LAL accessory products, EndoScan-V .
problems with endotoxin tests

Resultado da 21 de jan. de 2024 · Publicidade. Resultado Axé da Sorte. AO VIVO: Resultado completo será divulgado nesta página, no domingo, após a .
laboratory endotoxin analysis|problems with endotoxin tests